There is now abundant evidence that covid mRNA vaccines as well as more traditional 'killed" vaccines are effective in either preventing or modifying for the better, the disease manifestations of the dreaded SARS CoV-2 infection.
A new drug is usually approved by FDA or European regulators after robust animal and human volunteer studies demonstrate safety and efficacy in Phases 1 and 11 studies before more definitive Phase 111 studies show superiority to placebo when there is a genuine uncertainty or "equipoise" about absence of an effective preventive or therapeutic option, within the community of treating physicians.
Placebo refers to chemically inert substances that look, appear and taste similar to the active therapy and are used to mask subjects and investigators to the allocated treatment assigned in randomized controlled trials. Here subjects are assigned purely by chance to either active treatment or placebo to prevent selection, performance and detection bias.
In the case of covid-19, emergency authorization has been given by most robust regulatory bodies after rapidly performed clinical trials. Drug regulatory authority of Pakistan has approved some recent Chinese vaccine candidates based on trials in Pakistani volunteers.
Placebo controlled trials are considered ethical in the absence of any effective preventive or therapeutic option. Additionally these may be permissible in cases of terminally ill patients, where again no viable alternatives are available.
Lastly, if a trial is done where transient use of a placebo results in no major adverse effect, as long as an active therapy is not withheld subsequently, these are considered to be ethical. However, when a standard of care is available, a randomized controlled trial may only be performed with a standard therapy plus innovator drug versus standard care plus placebo. Once standard therapy is approved, superiority, bioequivalence studies or non-inferiority studies may be performed between two approved options.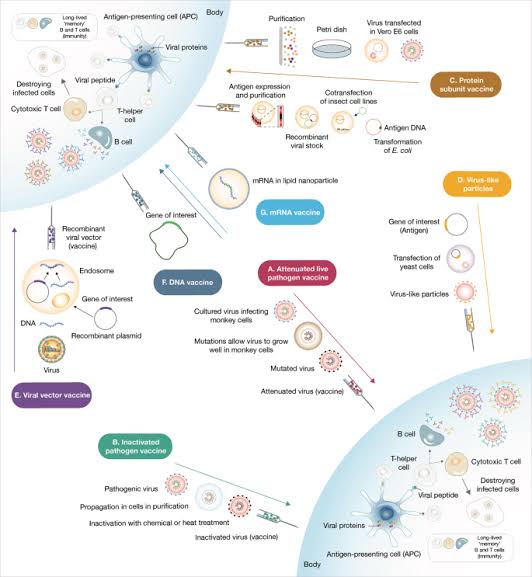
With a rush of sponsors making a beeline in Pakistan, I wonder if the National Bioethics Committee and various institutional review boards are looking at the pros and cons carefully in our potentially vulnerable volunteers being offered close to 16,000 Rupees for participating in these trials. This is a longstanding concern with the globalization of clinical trials in populations which either have a large base of vulnerable subjects because of financial aspects, potentially compromising autonomy or have a poor capacity to perform high quality and valid clinical trials because of absence of appropriate training in clinical research.
Should this aspect be brought up at our ministry overseeing the regulatory bodies, I propose for the medical community at large to address, and would encourage, a debate at the national level on the pros and cons of continued placebo controlled trials in the deadliest pandemic in the last one hundred years.
A new drug is usually approved by FDA or European regulators after robust animal and human volunteer studies demonstrate safety and efficacy in Phases 1 and 11 studies before more definitive Phase 111 studies show superiority to placebo when there is a genuine uncertainty or "equipoise" about absence of an effective preventive or therapeutic option, within the community of treating physicians.
Placebo refers to chemically inert substances that look, appear and taste similar to the active therapy and are used to mask subjects and investigators to the allocated treatment assigned in randomized controlled trials. Here subjects are assigned purely by chance to either active treatment or placebo to prevent selection, performance and detection bias.
In the case of covid-19, emergency authorization has been given by most robust regulatory bodies after rapidly performed clinical trials. Drug regulatory authority of Pakistan has approved some recent Chinese vaccine candidates based on trials in Pakistani volunteers.
Placebo controlled trials are considered ethical in the absence of any effective preventive or therapeutic option. Additionally these may be permissible in cases of terminally ill patients, where again no viable alternatives are available.
Lastly, if a trial is done where transient use of a placebo results in no major adverse effect, as long as an active therapy is not withheld subsequently, these are considered to be ethical. However, when a standard of care is available, a randomized controlled trial may only be performed with a standard therapy plus innovator drug versus standard care plus placebo. Once standard therapy is approved, superiority, bioequivalence studies or non-inferiority studies may be performed between two approved options.

With a rush of sponsors making a beeline in Pakistan, I wonder if the National Bioethics Committee and various institutional review boards are looking at the pros and cons carefully in our potentially vulnerable volunteers being offered close to 16,000 Rupees for participating in these trials. This is a longstanding concern with the globalization of clinical trials in populations which either have a large base of vulnerable subjects because of financial aspects, potentially compromising autonomy or have a poor capacity to perform high quality and valid clinical trials because of absence of appropriate training in clinical research.
Should this aspect be brought up at our ministry overseeing the regulatory bodies, I propose for the medical community at large to address, and would encourage, a debate at the national level on the pros and cons of continued placebo controlled trials in the deadliest pandemic in the last one hundred years.
